Press Release | AUG 20: NIH Funding and Moving to Trials
Honolulu, HI | AUG 20th – Oceanit’s ASSURE-19 test kit awarded NIH RADx funding, set to begin clinical trials.
Oceanit Laboratories’ ASSURE-19 rapid point-of-need COVID-19 test has been selected for continued support by the National Institutes of Health (NIH) Rapid Acceleration of Diagnostics (RADxSM) initiative, to fill the nation’s critical need for rapid, simple, and affordable COVID-19 testing. Initial work will focus on quickly bringing ASSURE-19 to market providing schools and airports with a simple saliva test. This phase will help with regulatory compliance and approval, as well as support for accelerating a ‘go to market’ timeline and scale manufacturing.
With Oahu under closure orders from August 20th for an additional 28 days, the need for rapid and persistent testing has become even greater.
The NIH RADx initiative was built to speed innovation in development, commercialization, and implementation of technologies for COVID-19 testing. Studies from Harvard University have estimated that up to 20 million tests are needed per day in order to safely reopen and re-mobilize the economy. These tests must be accurate, fast, easy-to-use, and widely accessible.
Oceanit submitted ASSURE to the NIH’s ‘Shark Tank’ in June. Along with several other funding recipients, Oceanit was chosen from a field of >7,500 applications. ASSURE’s fast-tracked development was built upon years of Artificial Intelligence work applied to creating the future of medicine – “Digital Medicine” – where guesswork is replaced by developing casual, mathematical design principals leveraging AI. This paradigm shift replaces “hunting and gathering” results with massive correlation of data to “engineering and designing” solutions.
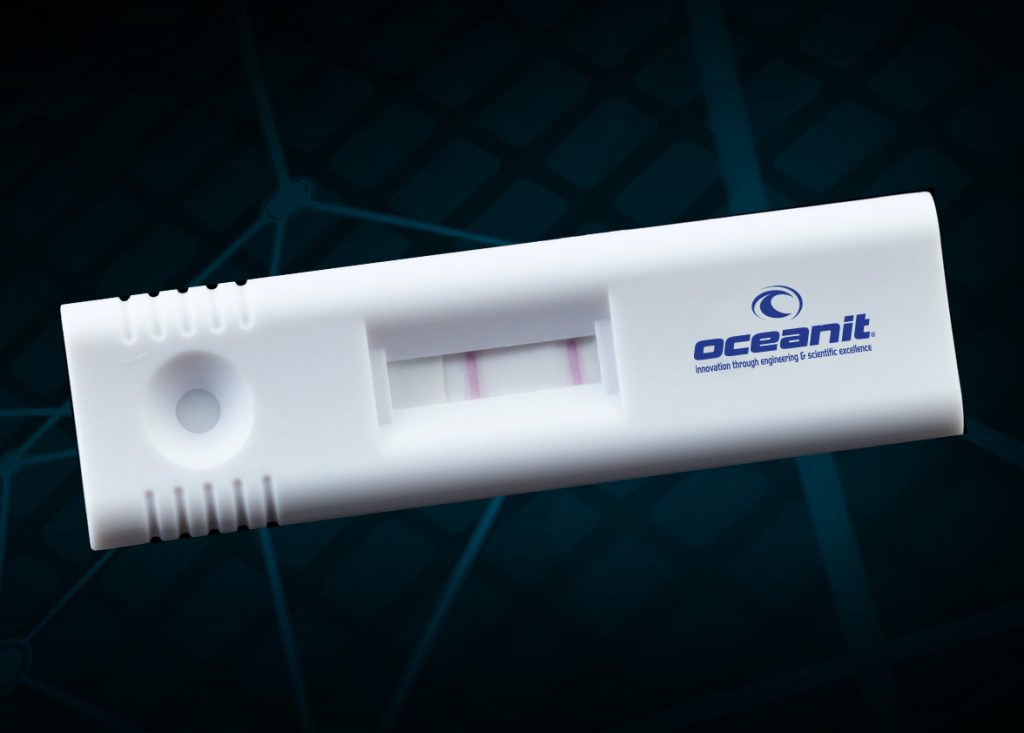
ASSURE-19 is a completely novel saliva diagnostic that requires no lab processing or equipment and no trained technicians. While other saliva tests are being deployed, ASSURE-19 is the only diagnostic that provides results within minutes, without needing any specialized processing equipment: designed as a “point-of-need” test built for mass production and widespread, possibly daily, for at-risk individuals even if they are pre-symptomatic or asymptomatic.
Oceanit will begin the US FDA data gathering process for ASSURE-19 this week. Using the data captured in two trials, Oceanit will then apply for FDA Emergency Use Authorization (EUA). The affordability, ease of use, and scalability of ASSURE-19 tests mean that testing could be made widely available, to all those at risk of exposure, not just those with symptoms. ASSURE-19 can be deployed without delays for results or due to lack of material supplies, both for Hawaii and for the US.
To learn more, contact assure-19@oceanit.com