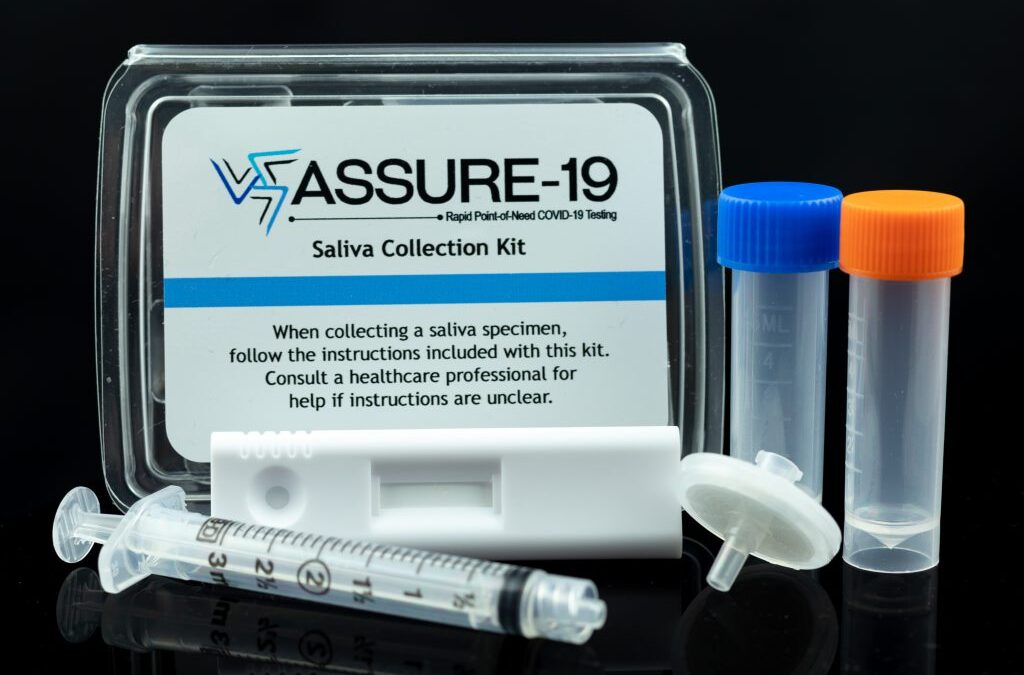
by Oceanit | Dec 28, 2020 | Local News
IN THE NEWS | Oceanit Progresses Towards FDA EUA Submission Oceanit has been hard at work to develop and perfect the ASSURE-19 rapid test for submission to the U.S. FDA for emergency use authorization. Hawaii News Now reconnected with Dr. Patrick Sullivan last week to...

by Oceanit | Nov 6, 2020 | General, National News
COVID-19 Cases Still Growing, Links to Academic Papers On October 24th, the United States tracked 78,702 new infections of Covid-19, a +32% 14-day change. The same day, 871 new COVID-related deaths were counted, this represents a +15% 14-day change. According to the...
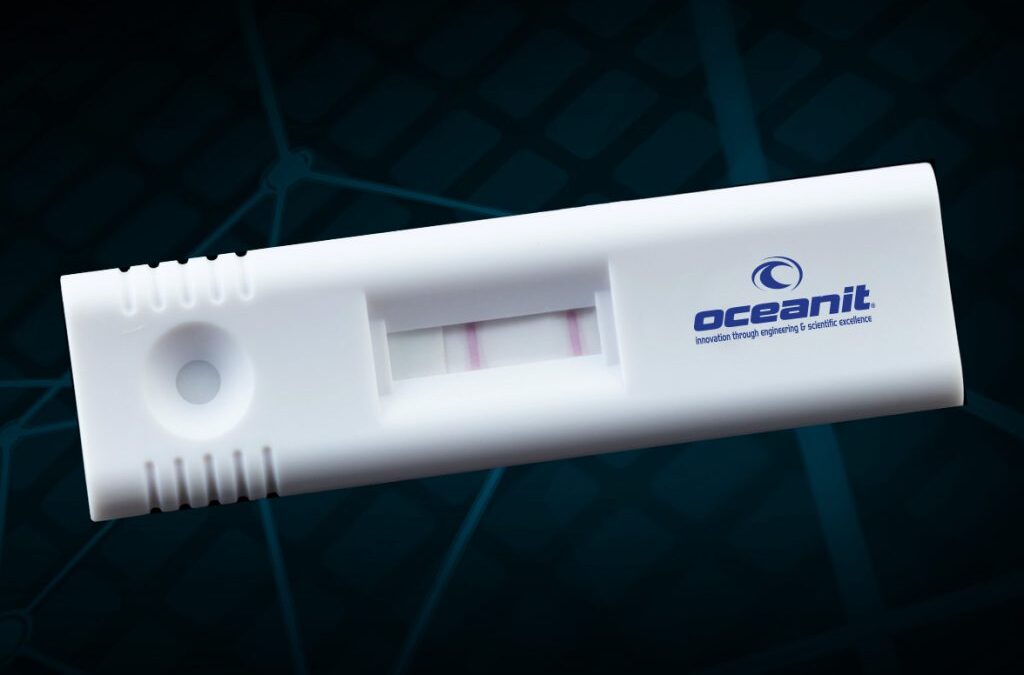
by Oceanit | Aug 19, 2020 | Government, Local News, National News
Press Release | AUG 20: NIH Funding and Moving to Trials Honolulu, HI | AUG 20th – Oceanit’s ASSURE-19 test kit awarded NIH RADx funding, set to begin clinical trials. Oceanit Laboratories’ ASSURE-19 rapid point-of-need COVID-19 test has been selected for...




