
by Oceanit | Apr 26, 2023 | Government, National News
PRESS RELEASE | Free ASSURE-100 Rapid Tests for Kūpuna and At-Risk Hawai’i Residents as Federal Support Ends Honolulu, HI April 26, 2023 | On May 11th, 2023 the U.S. federal government will officially end the COVID-19 Public Health Emergency (“PHE”) that began three...

by Oceanit | Feb 28, 2022 | Government, International News, National News
PRESS RELEASE | Oceanit Receives FDA Emergency Use Authorization for ASSURE-100 Rapid COVID-19 Tests FOR IMMEDIATE RELEASE ASSURE-100 Rapid COVID-19 Tests quickly and reliably detect all variants of SARS-CoV-2, including the delta and omicron. Simple, easy to use and...

by Oceanit | Oct 8, 2020 | Government, National News
PRESS RELEASE | National Institutes of Health Approves Next Round of ASSURE-19 Funding Under the RADx ‘Next’ Program Honolulu, HI | Oceanit Laboratories received approval from NIH for next round funding under the RADx initiative, receiving an additional $250,000 for...
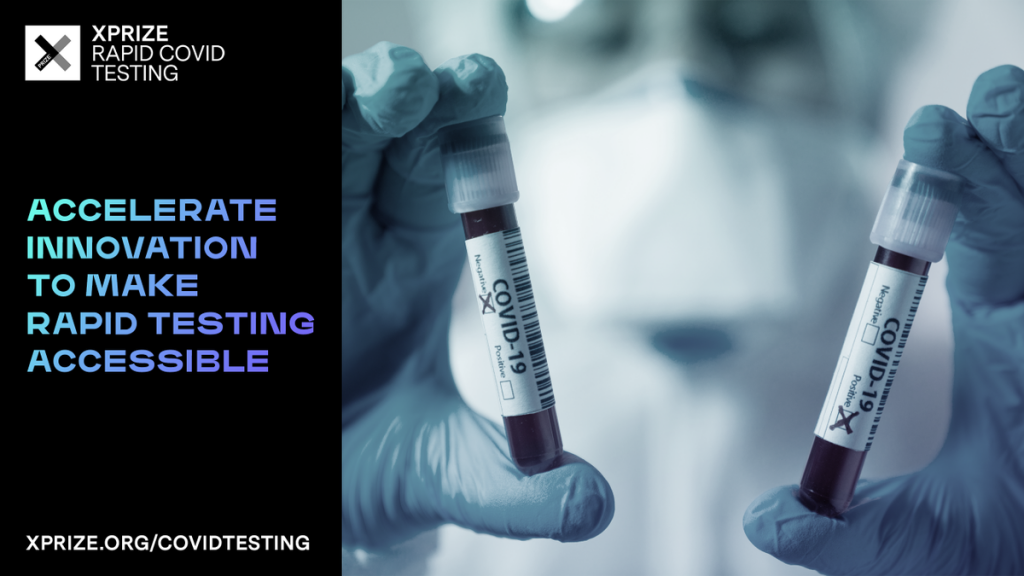
by Oceanit | Sep 16, 2020 | Future, International News
PRESS RELEASE | Oceanit Selected As a Semi-Finalist For the XPRIZE Rapid Covid Testing Competition Oceanit’s ASSURE-19 Covid-19 test was selected as a semi-finalist from a pool of nearly 700 teams from around the world competing in the XPRIZE Rapid Covid Testing...
by Oceanit | Aug 25, 2020 | General
PRESS RELEASE | ASSURE-19 Begins Clinical Testing to Acquire FDA Emergency Use Authorization Oceanit and the Queen’s Medical Center have started patient trials with ASSURE-19 rapid point-of-need Covid-19 tests as of Saturday, August 22. Oceanit is collaborating with...






