On May 11 the US government will end the COVID-19 Public Health Emergency covering free Covid-19 tests. In response, Oceanit plans to give free ASSURE-100 rapid tests to Hawaii Kūpuna and residents who are at high risk for adverse effects from Covid-19.
All posts tagged: update
ASSURE In the News

PRESS RELEASE | Free ASSURE-100 Rapid Tests for Kūpuna and At-Risk Hawai’i Residents as Federal Support Ends

IN THE NEWS | FDA Roundup: December 23, 2022
On December 23, the U.S. Food and Drug Administration provided an “at-a-glance summary” of news from around the agency, which included the over-the-counter EUA for ASSURE-100 Rapid COVID-19 At-Home Tests.

Hawaii News Now | Hawaii-Made Rapid COVID Test Gets Emergency FDA Approval
Hawaii News Now visited Oceanit office to interview the ASSURE team about the recent US FDA Emergency Use Authorization for the ASSURE-100 Rapid COVID-19 test

KITV4 | Oceanit Gets Emergency Clearance for Rapid COVID-19 Tests
KITV4 Island News visited Oceanit’s offices and labs yesterday to speak with Oceanit CEO, Dr. Patrick Sullivan about the newly authorized ASSURE-100 Rapid COVID-19 Test.
HPR | FDA Approves Emergency Use for First Rapid COVID-19 Test Developed in Hawaiʻi
Oceanit CEO, Dr. Patrick Sullivan joined Hawaii Public Radio host, Catherine Cruz to discuss the ASSURE-100 Rapid COVID-19 Test. Their ten minute discussion covered the research & development that went into ASSURE-100, the FDA EUA process, and what comes next.

JAMA Forum | FDA’s Policy Changes for COVID-19 At-Home Diagnostics—Implications for Addressing Other Infectious Diseases and Future Pandemics
In an article from December 23rd, 2021, Scott Gottlieb, MD from the American Enterprise Institute in Washington, DC discusses how the most enduring technological innovation of the pandemic so far may be the advent of accurate diagnostic tests
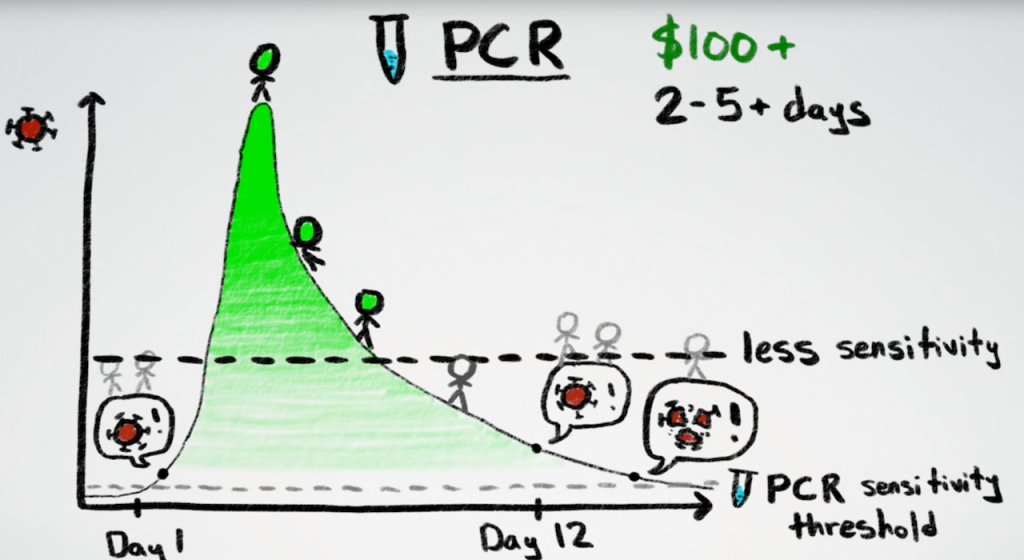
Despite Vaccines, the Need for Rapid COVID-19 Tests Remains
The need for rapid COVID-19 tests will certainly continue throughout 2021, despite the great progress made on vaccines. Shots will reach everyone who wants them, sooner or later, but perhaps not as quickly as we may like. Rapid tests could be made available right now, without a prescription, so we don’t have to wait months for the vaccine to arrive.

The Aloha Protocol For a Safer Reopening with ASSURE-19
As Oceanit moves ahead with in-vitro and in-vivo testing for the ASSURE-19 rapid point-of-need test kits, Hawaii is seeing high, sustained counts for new covid-19 cases each day. Check out the latest articles and coverage of ASSURE-19 from the Washington Post, Hawaii Public Radio, and Honolulu Star Advertiser.
PRESS RELEASE | ASSURE-19 Begins Clinical Testing to Acquire FDA Emergency Use Authorization
Oceanit and the Queen’s Medical Center have started patient trials with ASSURE-19 rapid point-of-need covid-19 tests as of Saturday, August 22. Oceanit is collaborating with Dr. Todd B. Seto, QMC’s director of academic affairs and research, to obtain critical test data. This is the beginning of the journey to acquire US Food and Drug Administration Emergency Use Authorization (EUA).

ASSURE-19 Featured on Hawaii News Now, Honolulu Star Advertiser, and Pacific Business News
Oceanit is focusing on quickly bringing ASSURE-19 to market, organizing manufacturing so that we can provide schools, emergency responders, and airports with a simple saliva test to make high-risk situations safer. This phase of funding and work will help with regulatory compliance and approval, as well as support for accelerating a ‘go to market’ timeline and scale manufacturing.