
by Oceanit | Mar 2, 2022 | Local News
KITV4 | Oceanit Gets Emergency Clearance for Rapid COVID-19 Tests KITV4 Island News visited Oceanit’s offices and labs yesterday to speak with Oceanit CEO, Dr. Patrick Sullivan about the newly authorized ASSURE-100 Rapid COVID-19 Test. Reporter Kristen Consillio...
by Oceanit | Mar 2, 2022 | Local News
HPR | FDA Approves Emergency Use for First Rapid COVID-19 Test Developed in Hawaiʻi Oceanit CEO, Dr. Patrick Sullivan joined Hawaii Public Radio host, Catherine Cruz to discuss the ASSURE-100 Rapid COVID-19 Test. Their ten minute discussion covered the research &...

by Oceanit | Jan 13, 2022 | National News
JAMA Forum | FDA’s Policy Changes for COVID-19 At-Home Diagnostics—Implications for Addressing Other Infectious Diseases and Future Pandemics In an article from December 23rd, 2021, Scott Gottlieb, MD from the American Enterprise Institute in Washington, DC discussed...
by Oceanit | Jan 15, 2021 | General
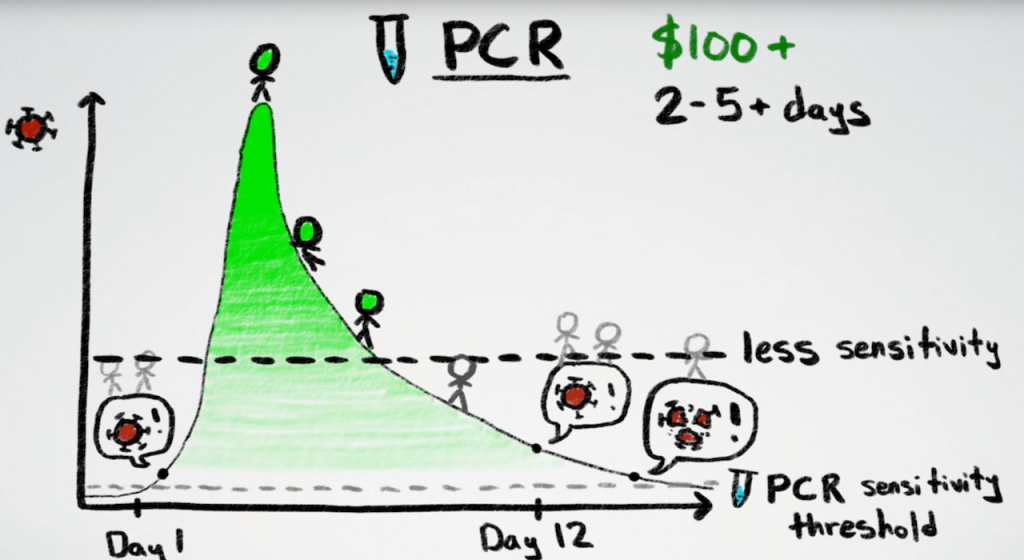
Despite Vaccines, the Need for Rapid COVID-19 Tests Remains In December 2020, as vaccines for SARS-CoV-2 began rolling out across the country and around the world, the New York Times released a vaccine timeline calculator to help readers determine how long it could be...

by Oceanit | Sep 2, 2020 | Future, Government, Local News, National News
The Aloha Protocol For a Safer Reopening with ASSURE-19 As Oceanit moves ahead with in-vitro and in-vivo testing for the ASSURE-19 rapid point-of-need test kits, Hawaii is seeing high, sustained counts for new covid-19 cases each day. Oahu reentered lockdown last week...






