by Oceanit | Jan 29, 2025 | Education, General
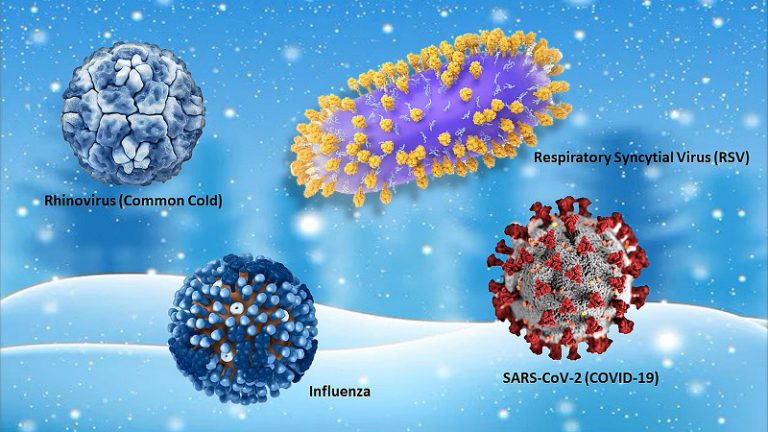
Surge in Respiratory Illnesses: California’s New Bird Flu Outbreak, Rising HMPV Cases, and Persistent COVID-19 In recent months, a notable increase in respiratory illnesses has been observed, raising concerns among health professionals and the public alike. This surge...

by Oceanit | Jan 22, 2025 | General, Government
RFK Jr. and H5N1: What to Expect in a Bird Flu Pandemic The H5N1 bird flu, long considered a significant pandemic threat, has been making headlines in recent months. As concerns grow about its potential to cross more effectively into human populations, questions arise...




