
by Oceanit | Jan 21, 2025 | Future, Government
COVID-19 Updates: What to Know Under the New Trump Administration As a new administration takes office, COVID-19 remains a public health priority. Understanding how policies and resources are evolving under this leadership is essential for navigating the ongoing...

by Oceanit | Jan 17, 2025 | Education, General, Government
CDC Guidance for the 2024-2025 Respiratory Virus Season As the 2024-2025 respiratory virus season continues, the Centers for Disease Control and Prevention (CDC) has issued updated recommendations to help individuals navigate the overlapping threats of COVID-19,...
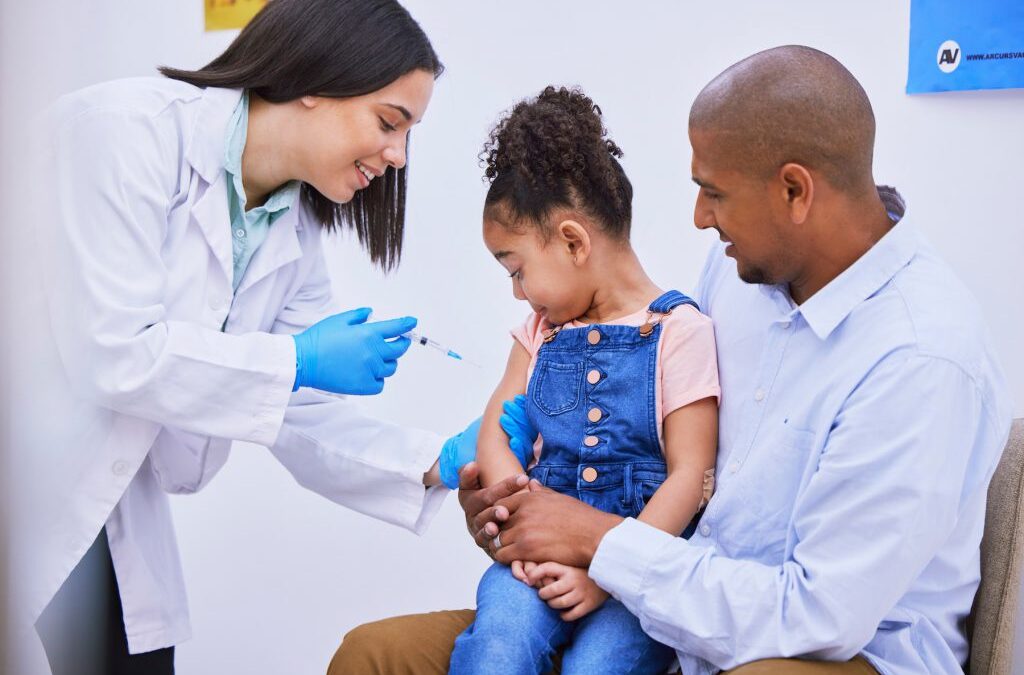
by Oceanit | Jan 16, 2025 | General, Government
Understanding the FDA’s Role in COVID-19 Rapid Tests and Vaccines The Food and Drug Administration (FDA) has played a critical role in the fight against COVID-19. From evaluating the safety and efficacy of vaccines to authorizing rapid testing methods, the agency has...

by Oceanit | Jan 16, 2025 | Future, Government, National News
COVID-19 and the Trump Administration: Policies and Plans for 2025 As President-elect Donald Trump prepares to take office in January 2025, COVID-19 remains a significant public health challenge. The incoming administration’s policies will shape the nation’s pandemic...

by Oceanit | Jan 10, 2025 | Future, Government, History
Preparing for Future Global Pandemics: Science and Policy The COVID-19 pandemic served as a wake-up call for the global community, exposing vulnerabilities in healthcare systems, supply chains, and public health policies. As the world recovers from the impact of...

by Oceanit | Jan 9, 2025 | Education, General, Government
The Evolution of mRNA Vaccines: COVID-19 and the Future The COVID-19 pandemic highlighted the transformative potential of mRNA vaccines, which became a cornerstone of global vaccination efforts. Developed at unprecedented speed, these vaccines saved millions of lives...







