
by Oceanit | Mar 17, 2025 | Education, Future, National News
Post-Pandemic School Policies: Attendance and Health Guidelines In the wake of the COVID-19 pandemic, U.S. schools have implemented new policies to ensure the safety and well-being of students and staff. These measures, guided by recommendations from the Centers for...

by Oceanit | Mar 14, 2025 | Future, General
Latest Research on Long COVID and Its Long-Term Effects on COVID-19 Survivors As the world continues to navigate the aftermath of the COVID-19 pandemic, long COVID has emerged as a significant public health concern. Recent studies and reports shed light on the...

by Oceanit | Mar 13, 2025 | Future, General
Envisioning Public Health in 2045: Lessons from COVID-19, mRNA Vaccines, and Pandemic Responses The COVID-19 pandemic has indelibly transformed the landscape of public health, catalyzing innovations and strategies that will shape our approach to health crises over the...
by Oceanit | Mar 13, 2025 | Education, Future
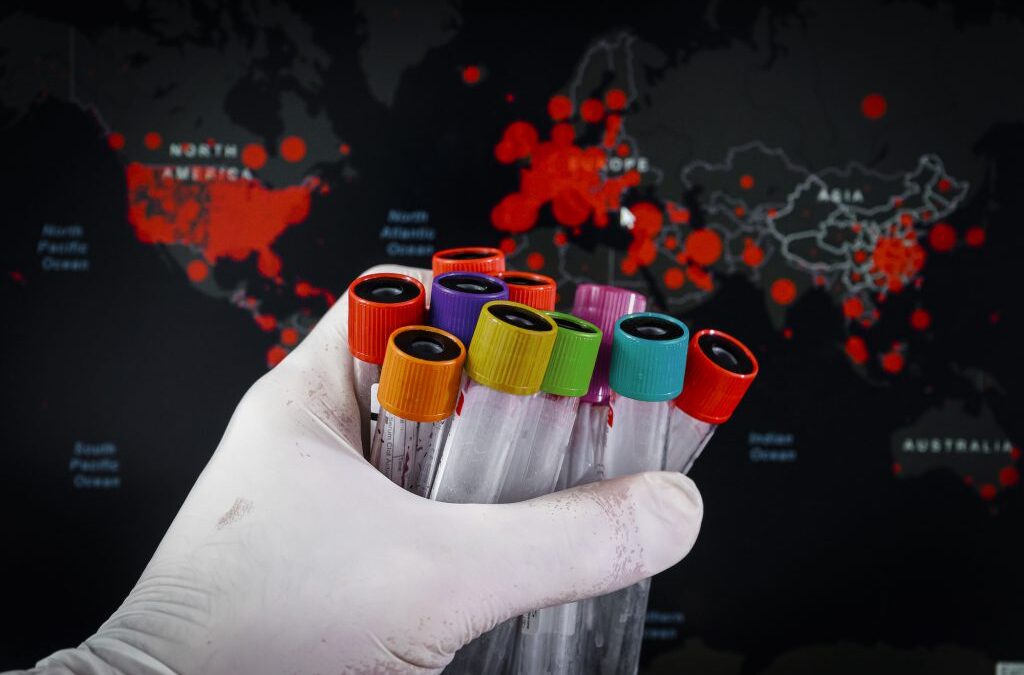
Staying Ahead of Evolving COVID-19 Variants: How Scientists Track SARS-CoV-2 Mutations The rapid evolution of SARS-CoV-2, the virus responsible for COVID-19, has continually challenged global public health efforts. A recent article from NPR delves into how scientists...

by Oceanit | Mar 13, 2025 | Future, General
Seasonal Viral Infections Decline: Understanding the Quad-demic Cycle of Flu, COVID-19, and RSV As of March 13, 2025, the United States is witnessing a notable decline in seasonal viral infections, particularly influenza (flu), COVID-19, and respiratory syncytial...

by Oceanit | Mar 12, 2025 | Future, General
COVID-19 Variants: Evolution Pace, Booster Shots, and Infectiousness Since its emergence, SARS-CoV-2, the virus responsible for COVID-19, has undergone continuous evolution, leading to the development of multiple variants. Understanding the pace at which these...







