INTRODUCING THE ASSURE-100 RAPID COVID-19 HOME TEST
On December 22, 2022, the ASSURE-100 Rapid COVID-19 Home Test received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for at-home use.
The ASSURE-100 is designed for the qualitative detection of the SARS-CoV-2 nucleocapsid protein antigen using direct shallow nasal swab samples. This over-the-counter (OTC) test does not require a prescription from a healthcare provider, making it highly accessible for home use.
Compared to traditional polymerase chain reaction (PCR) tests, rapid antigen tests like the ASSURE-100 are faster and more affordable. These tests provide individuals with a simple, low-cost method to protect family members, classmates, and coworkers, especially during high-risk periods such as the holidays. By enabling users to quickly determine their infection status and monitor their health over time, at-home rapid tests play a critical role in managing the spread of COVID-19.
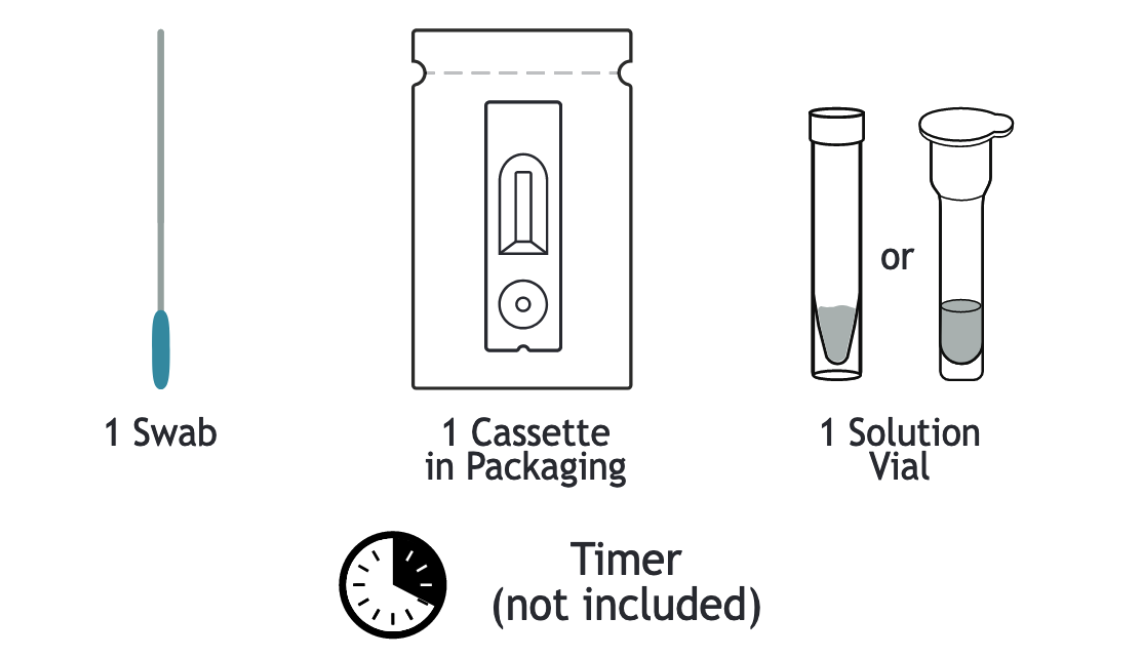
ASSURE-100 Rapid COVID-19 At-Home Test Kit Contents
A New Normal
Protect Yourself and Your Family with ASSURE-100 During Cold and Flu Season! Although many aspects of daily life are returning to normal in our communities, the fight against COVID-19 is not over. As we enter the winter months—with flu, respiratory syncytial virus (RSV), and COVID-19 cases on the rise—it’s crucial to maintain proactive health measures. We encourage everyone to stay up to date with their COVID-19 vaccinations to protect themselves, their families, and the community at large.
The FDA EUA for the ASSURE-100 Rapid COVID-19 Home Test facilitates serial asymptomatic testing, allowing individuals to regularly monitor their infection status at home. This test offers convenience and peace of mind, with a simple design that includes only three components: a nasal swab, a solution vial, and a test cassette. The ASSURE-100 delivers results in just 20 minutes, making it both affordable and easy to use.
Clinical trials for the ASSURE-100 Rapid COVID-19 Home Test demonstrated excellent performance, with a sensitivity of 84.7% (positive percent agreement) and 100% specificity (negative percent agreement) in patients suspected of COVID-19 infection. This high level of accuracy makes it a reliable option for self-testing and monitoring health at home.
How does the ASSURE-100 home test work?
The ASSURE-100 Rapid COVID-19 Home Test detects SARS-CoV-2 antigens directly from anterior nares swab specimens – a shallow nasal swab. The specimen is then plunged in a specialize buffer solution, before the solution is applied to a lateral flow assay (LFA). ASSURE-100 provides accurate and reliable results in 20 minutes without a prescription. The test is simple, fast, accurate and affordable, and was designed by scientists and engineers in Hawaii and is distributed worldwide.
ASSURE-100 Rapid COVID-19 Home Test Kit Instructions For Use
FDA EUA Settings for ASSURE-100 Rapid COVID-19 Home Tests
The ASSURE-100 Rapid COVID-19 Home Test EUA authorized settings include: Home, H, M, W:
- Home Test – Authorized as an At-Home COVID-19 antigen test
- H – Laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet requirements to perform high complexity tests.
- M – Laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet requirements to perform moderate complexity tests.
- W – Patient care settings operating under a CLIA Certificate of Waiver.
FDA Informational Links
- To learn more, visit: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-antigen-diagnostic-tests-sars-cov-2#IndividualEUAs
- At-Home Test taking: https://www.fda.gov/medical-devices/safety-communications/home-covid-19-antigen-tests-take-steps-reduce-your-risk-false-negative-results-fda-safety
FAQs
1. How does the ASSURE-100 Rapid COVID-19 Home Test Work?
The ASSURE-100 Rapid COVID-19 Home Test detects SARS-CoV-2 antigens directly from anterior nares swab specimens. It provides accurate and reliable results within 20 minutes, allowing for widespread COVID-19 testing of individuals with or without symptoms when tested serially. The ASSURE-100 Rapid COVID-19 Home Test is simple, fast, accurate and affordable, enabling a safe reopening for schools, businesses, and organizations around the world. ASSURE-100 was designed by scientists and engineers in Hawaii and is distributed worldwide.
2. What is ASSURE-100 Rapid COVID-19 Home Test?
The ASSURE-100 Rapid COVID-19 Home Test is a rapid antigen test that uses an anterior nasal specimen to detect COVID-19, the disease caused by the SARS-CoV-2 virus. ASSURE is an acronym for: Accelerated Sensor Solution for Urgent Response to Epidemics.
3. Where was ASSURE-100 Rapid COVID-19 Home Test Developed?
The ASSURE technology and ASSURE-100 rapid COVID-19 test was developed by Oceanit in Hawaiʻi and was put through testing and clinical trials in Hawaii and in other states across the US.
4. How do ASSURE-100 Rapid COVID-19 Home tests work?
ASSURE-100 Rapid COVID-19 Home Test uses shallow nasal specimens to detect COVID-19 using a lateral flow assay (LFA) test cassette. A shallow anterior nasal swab specimen is mixed with buffer solution and then poured into the well on the test cassette. ASSURE tests are visually read, and do not require an external device to interpret results.
5. Does ASSURE-100 Rapid COVID-19 Home Test detect Omicron variants?
Yes, the ASSURE-100 Rapid COVID-19 Home Test clinical trial included BA.2.12.1 and BA.2 variant samples. Additionally, the ASSURE-100 test was evaluated in a dilution series of clinical specimens positive for the Omicron variants BA.5 and BE.1. This testing was conducted by National Institutes of Health (NIH) as a component of the Rapid Acceleration of Diagnostics (RADx) initiative. Results indicate that ASSURE-100 detects both BA.5 and BE.1 variants.
6. How quickly will ASSURE-100 Rapid COVID-19 Home Test detect a result if positive?
Results should be read at 20 minutes.
6. Where can I get ASSURE-100 Rapid COVID-19 Home tests?
ASSURE-100 Rapid COVID-19 Home Test can be purchased directly by contacting Oceanit Foundry.
This product has not been FDA cleared or approved, but has been authorized by FDA under an EUA; this product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens; and, the emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb- 3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
